
PTAB Finds That a Mixture of Three Milk Sugars is a Product of Nature
The Patent Trial and Appeal Board recently affirmed the 35 U.S.C. § 101 rejection of patent claims for a mixture of three human milk oligosaccharides (HMOs), finding the ternary mixture to be ineligible for patenting.1 This decision provides helpful insight into how compositions involving naturally occurring constituents are evaluated by the Board, and highlights ongoing challenges that inventors and patent applicants may face, even when claiming compositions that are developed in a laboratory.
The Technology
Human milk is primarily water, but also contains sugars, fats, proteins, and minerals. Certain sugars, known as HMOs, are of particular interest for their potential health properties. At least 130 HMOs are known to be present in milk. The invention involved Glycom’s combination of three specific HMOs, namely 6′-SL, LNnT, and LST c. This three-part mixture was found to have anti-infective properties, rendering it useful for treating viral or bacterial infections.
The claimed mixture was limited to these three HMOs only, and did not include other components, making it distinctly different from naturally occurring milk. Furthermore, the mixture was prepared using a laboratory enzymatic process. So how did the Patent Office conclude that it was a product of nature?
The § 101 Rejection
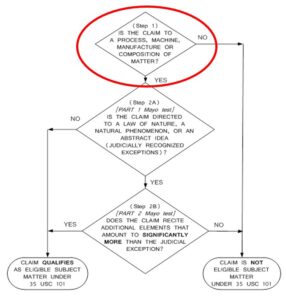
Following the USPTO’s 2014 Interim Guidance on Patent Subject Matter Eligibility (2014 IEG) depicted in the flow chart below, under Step 1 the Examiner first determined that the claims were directed to one of the four statutory categories, namely as a “composition of matter.”2
Under revised Step 2A Prong One of Revised Patent Subject Matter Eligibility Guidance (2019 PEG) shown below, the claim was found to recite a judicial exception, namely a natural phenomenon.
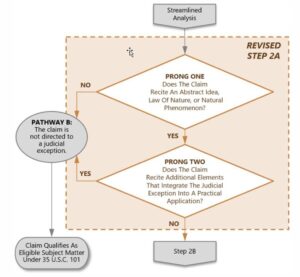
In assessing the nature-based product, the examiner found that none of the claimed HMO components exhibited any change in structure or function with respect to how they occur in nature. According to the USPTO guidelines, if a nature-based product as presented in the claim has markedly different characteristics (MDC test) as compared to any naturally occurring counterparts, there is no judicial exception and the claim is eligible (Step 2A Prong One: NO), whereas if the nature-based product does not have markedly different characteristics, then the claim is directed to the “product of nature” judicial exception (Step 2A Prong One: YES) and the analysis proceeds to Step 2A Prong Two.
With reference to Step 2A Prong Two, the examiner found that the judicial exception was not integrated into a practical exception, because the claims did not recite anything beyond the HMOs themselves, and any intended use did not accomplish such an integration (Step 2A Prong Two: NO).
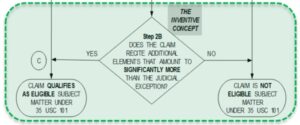
With reference to Step 2B, the examiner found that the claims as a whole did not recite additional elements amounting to something more than the judicial exception (Step 2B: NO), and therefore the claims were not eligible under § 101.
The Board’s Analysis
To begin, the Board agreed with the examiner that the claims recited a composition of matter, thus satisfying Step 1 of the Guidance. The Board also agreed that the claims were directed to a judicial exception at Step 2A Prong One, noting that “[w]hether the HMOs are isolated or subsequently replicated outside the human is irrelevant to whether they are found in nature.”
With regard to the markedly different characteristics (MDC) analysis at Step 2A Prong One, the Board noted that the application specification itself said that “[i]n some cases, the dosage will be at a concentration similar to that found for 6’-SL, LNnT and/or LST c in human breast milk.”
Relatedly, the Board emphasized that “the claims provide no ranges or specify any concentration of the HMOs, leaving the claims subject to the broadest interpretation in light of the Specification.” Accordingly, the Board concluded that the claims could be construed to encompass dosages as low as the levels of HMOs found in natural milk, and therefore the claims recited subject matter that was not markedly different from what is found in nature.
The Board also indicated that the Appellant did not meet the burden of showing a markedly different characteristic sufficient to warrant patent eligibility.
In sum, the Board’s analysis ended at Step 2A Prong One, and did not reach Step 2A Prong Two or Step 2B.
Key Takeaways for Patent Attorneys and Agents
When working with inventions that involve a mixture of natural products, it can be helpful to specify in the claims how the mixture components are different from the way in which they may occur naturally. Relying on the fact that the components have been isolated, purified, or synthesized may not be enough to establish patent eligibility. Consider drafting the patent application with the following guidelines in mind:
- Include specific quantitative descriptions (e.g. concentrations, amounts, related ranges) of mixture components
- Provide evidence of unique or improved biological/pharmacological functionality for the mixture
PERA Implications
Under the currently proposed language of the Patent Eligibility Restoration Act (PERA, Senate Bill 2140), the milk sugar mixture claims that were found ineligible by the Board would likely have been found to meet the patent eligibility requirement (e.g. pursuant to §§101(a), (b)(2)(A)).
Related Reading
As noted above, the Board placed the burden on the Applicant to show that the claimed subject matter markedly different characteristics as compared to a naturally occurring mixture (as part of the Step 2A Prong One analysis). Compare this result with the Board’s position in Ex parte Ma, where the Board seemed to implicitly put the burden of proof on the examiner to show that the claimed formulation lacked markedly different characteristics as compared to a product of nature (also as part of the Step 2A Prong One analysis).
1 Ex parte Champion (Appeal 2023-003725; November 6, 2024)
2 In Diamond v. Chakrabarty, 447 U.S. 303 (1980), the Supreme Court confirmed that the term “composition of matter” refers to “all compositions of two or more substances and all composite articles, whether they be the results of chemical union, or of mechanical mixture, or whether they be gases, fluids, powders or solids.”